
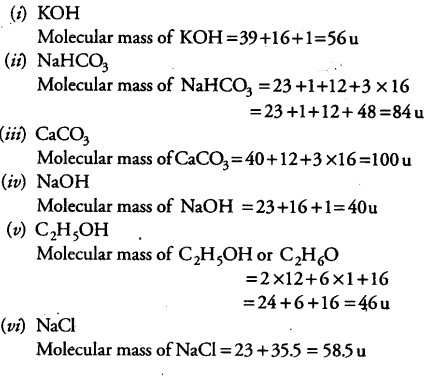
in the laboratory it is used as a preservative for specimens. Apart from that, there are medical applications of isopropyl alcohol such as the production of rubbing alcohol, hand sanitizers, etc. There are various uses of isopropyl alcohol used as a solvent for nonpolar compounds because isopropyl alcohol is moderately polar. The hydrogenation of acetone gives isopropyl alcohol in the process of Raney nickel catalyst. Indirect hydration includes the reaction between propene and sulfuric acid, which gives a mixture of sulfate esters. The production is done under high pressures and in the presence of an acidic catalyst. The reaction can be carried out in either the liquid phase or in the gas phase. In the direct hydration method, propene and water react with each other. There are three main ways of producing isopropanol This compound is an isomer of 1-propanol. The melting point is -88☌ and the boiling point is 108☌. The molar mass of isopropanol is 60 g/mol. Our calculated value is much lower than this due to the loss of heat energy to the surroundings such as the beaker or the air, as well as incomplete combustion.Figure 02: Molecular Structure of Isopropanol The accepted data book value for the combustion of ethanol is -1367kJ/mol. We can now calculate the molar enthalpy change for the combustion of ethanol using the expression: Therefore, 0.0130 moles of ethanol burned to produce 12.810kJ of energy. To calculate the value for △H, the value for Q in J must be converted in kJ by dividing the value by 1000. From our calculations we know that 0.0130 moles of ethanol burned to produced 12810J of energy. The molar enthalpy change tells us the amount of energy released by 1 mole of the substance.
The number of moles of ethanol burned is therefore: The molar mass for ethanol is calculated therefore as (2 x 12) + (5 x 1) + 16 + 1 = 46.0g/mol. The chemical formula of ethanol is C 2H 5OH. The molar mass of a substance is calculated by adding up the relative atomic masses of all elements in the substance. We can now calculate the number of moles of ethanol burned using the equation: Therefore, 0.6g of fuel releases 12810 joules of energy. To calculate the mass of ethanol burned therefore we subtract the final mass from the starting mass.Ġ.6g of ethanol was burned in this combustion reaction. The difference in mass is therefore the mass of ethanol burned. The masses of the spirit burner before and after heating differ due to the ethanol undergoing a combustion reaction and reacting with oxygen to produce carbon dioxide and water. To calculate the number of moles of ethanol burned, we firstly need to calculate the mass of ethanol used in the reaction. Calculate the molar enthalpy change for the combustion of ethanol. The heat energy change for the reaction was 12810J. After heating the mass was measured again and recorded as 68.15g. The mass of the ethanol, spirit burner and the lid before heating was measured as 68.75g. In worked example 1 from the previous section, we calculated that 12810J of heat energy was transferred to the water from the combustion of the ethanol in the spirit burner.īy adding some detail about the amount of ethanol that was burned we can calculate the molar enthalpy change for the combustion of ethanol, as shown in the next example. DH is given in kJ/mol and Q is given in J.
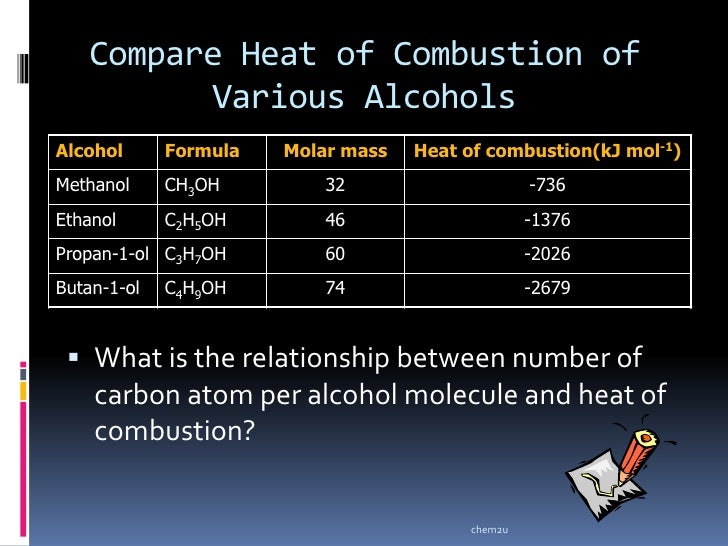
Notice that the units for △H and Q are different.

The number of moles of the substance that reacted can be calculated using the equation: N is the number of moles of the substance that reacted (mol) △H cannot be measured directly but can be calculated from the heat energy change using the expression: The amount of heat energy absorbed or released for every mole of the substance which reacts is known as the molar enthalpy change and is represented as △H. Although the heat energy change (Q) tells us the amount of heat energy transferred to or absorbed from the surroundings, it does not tell us the amount of heat energy absorbed or released for every mole of the substance which reacts.


 0 kommentar(er)
0 kommentar(er)
